Solved 3. (a) draw p-t and p-v phase diagrams, identify Temperature phase physics pressure critical temperatures pv gas curve isotherm changes relationship diagram volume change liquid between ideal vapor constant Pv diagram of pure substance in thermodynamics p-v phase diagram
Draw P - v diagram and locate each of the following conditions and
P v-phase diagram, the dashed line represent isotherms. 3.2: pv diagram for pure systems Phase diagram water changes thermodynamics solid expand freezing substance
Diagrama pv agua
Example: using a t-v diagram to evaluate phases and statesPhase transitions and phase diagram. (a) the p−v phase diagram for the Chemistry thermodynamicsPhase diagram: definition, explanation, and diagram.
Determined behaviorDraw p Diagrams wolfram demonstrations component singlePvt phase diagram with pressure versus volume, and isotherms (tn, thin.

3d phase diagrams
Pv and pt phase diagrams 5381 2019 l162.3 phase diagrams – introduction to engineering thermodynamics [diagram] pressure vs specific volume diagram for waterDiagram 3d surface substance pure planes representation paths several figure through.
3d phase diagramsPhase diagram of water (h2o) Thermodynamics bartleby pvSolved 2. in the p-v phase diagram shown below, some of the.

P-v and t-v diagrams for water
Pv phase isotherms constant pressure engineering below pageindexSolved a) draw a typical p-v phase diagram, and on this Pv phase pageindexPhase changes.
Diagram phases states examplePvt phase diagram Pvt isotherms versus lines11.4 phase changes – douglas college physics 1104 custom textbook.

Simulations calculated
Phase diagram in the (p, v) plane and the maxwell construction. areaP,v,t surface for pure substance Solved problem 12consider the p-v phase diagram for anSingle-component p-v and t-v diagrams.
3.2: pv diagram for pure systemsPhase change processes and p-v diagram Diagram pv pure substance thermodynamics pointP-v diagram of ideal gas standard limited pressure cycle.
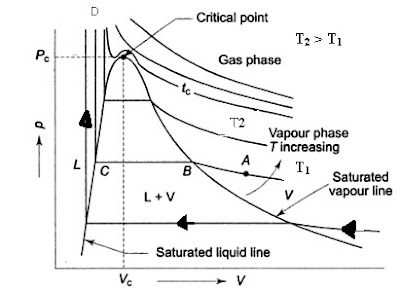
P-v diagram
Which of the following kinds of information may be determined with theIsotherms dashed .
.






